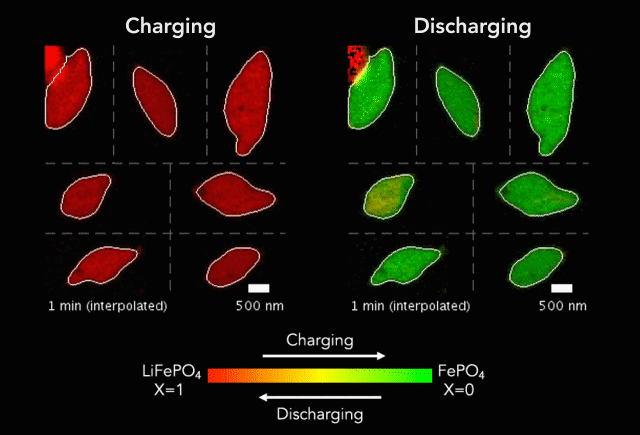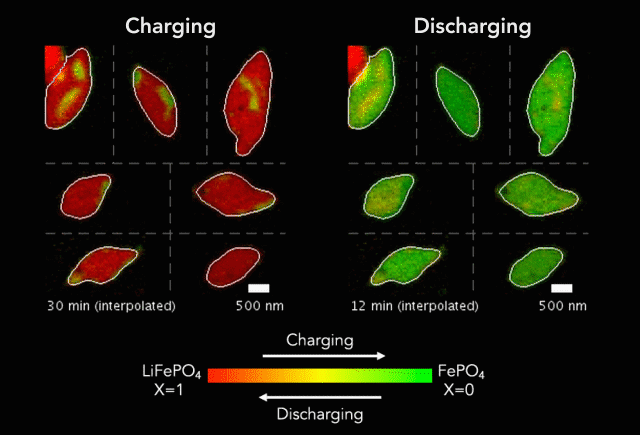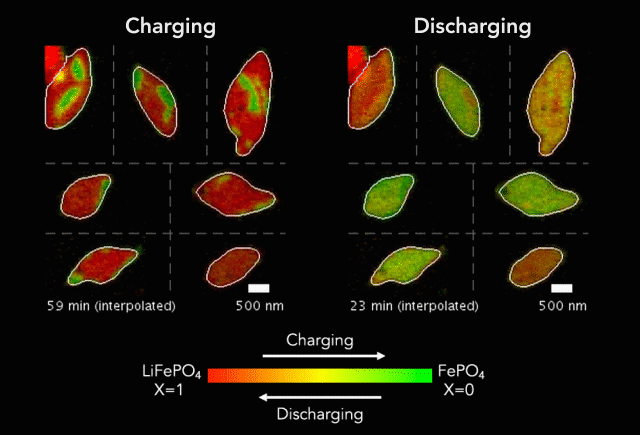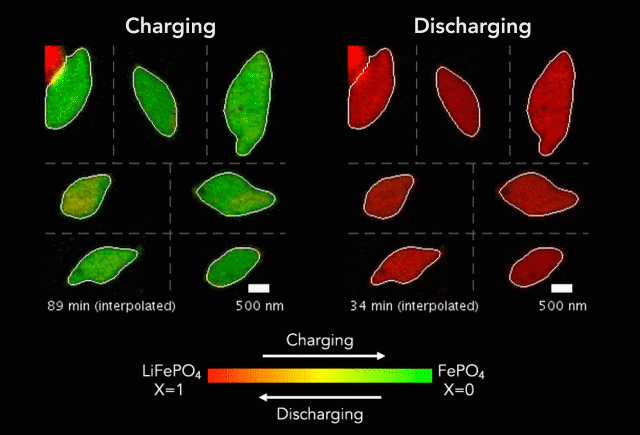
Comparison of (a) simulated and (b)-(c) observed lithiated diamond cores in LixFePO4 single particles.
My postdoc with Prof. Martin Bazant officially ended 6 years ago, but it’s still generating research papers! A paper that we wrote was just published at Electrochemistry Communications, and is available for free at this link until October 28.
Over that time we had been following the progress in advanced microscopy techniques applied to battery materials, and realized that our model of lithium iron phosphate captured many of the features researchers were imaging. The model helped resolve conflicting reports from different researchers who looked at a variety of different size LiFePO4 crystals. The smallest crystals at the nanoscale are controlled by their surface properties, while micron sized crystals are dominated by elastic energy. Just by running larger simulations we observed the transition that experimentalists were imaging. After a bunch of time invested on nights and weekends, this paper is finally finished.